Thermal oxidizers are industrial air pollution control devices that are used to treat exhaust gasses from various manufacturing processes. They work by heating contaminated air to high temperatures, causing the pollutants to break down into harmless byproducts. Read More…
Anguil Environmental provides highly-engineered, environmental equipment and service solutions that help clients solve complex industrial air pollution control and wastewater treatment challenges across the globe. Anguil air pollution control systems include thermal and catalytic oxidation technologies for compliance with VOC, HAP and odor regulations.
Dürr is a leading global supplier of environmental solutions and engineered products tailored to meet customers' industrial process requirements. We offer a complete portfolio of air pollution control technologies including scrubbers, wet electrostatic precipitators, thermal and catalytic oxidizers, and solvent recovery systems.
The CMM Group provides design and build, and technical engineering services for VOC emission control, odor abatement solutions and energy recovery systems. CMM Aftermarket Services team provides preventive maintenance and inspection services, controls upgrades, retrofit and rebuild services to extend the life of existing equipment. For small or large, complex projects, The CMM Group’s extensive ...

Pollution Systems designs, manufactures and installs highly reliable industrial air pollution control equipment. We offer Thermal Oxidizers, Catalytic Oxidizers, Regenerative Thermal Oxidizers, Direct Fired Oxidizers, Enclosed Flares, Gas Scrubbers, Particulate Scrubbers, Rotor Concentrators and Heat Recovery Systems. On-site services through our dedicated service company include equipment...
Meet stringent environmental regulations with Ducon's complete line of the most advanced air pollution control equipment: cyclones, scrubbers, incinerators, electrostatic precipitators, activated carbon absorbers, gas absorption towers, flue gas desulfurization, chemical strippers, NOx & VOC Control, etc.
More Thermal Oxidizer Manufacturers
Components of Thermal Oxidizers
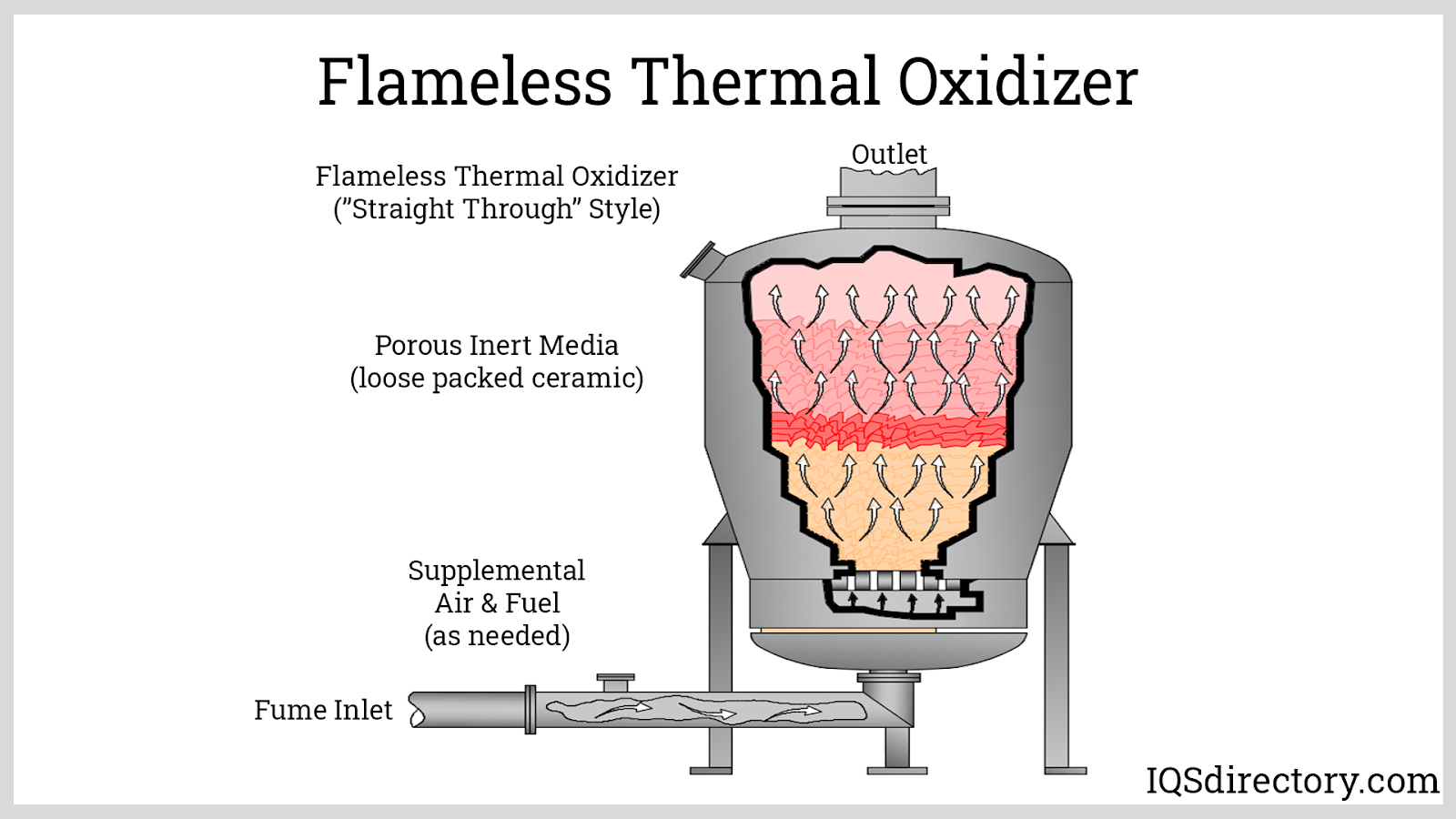
A thermal oxidizer typically consists of three main components: the combustion chamber, the burner system, and the exhaust system. In the combustion chamber, contaminated air is introduced and mixed with a fuel source, usually natural gas or propane, to initiate the combustion process. The burner system provides the heat necessary to sustain the high temperatures required for the thermal oxidation process to occur. The exhaust system is responsible for removing the treated air and directing it back into the environment.
Variations in Thermal Oxidizers
There are several variations of thermal oxidizers that differ in design or component selection. Regenerative thermal oxidizers (RTOs) are a popular variation that use ceramic beds to capture and recycle the heat generated by the combustion process. Recuperative thermal oxidizers (RPOs) use heat exchangers to preheat incoming contaminated air with the heat generated from the outgoing treated air. Direct-fired thermal oxidizers (DFTOs) directly combust the contaminated air without any heat exchange or recuperation, making them simpler but less efficient than the other variations. The choice of variation depends on the specific application and the required emission reduction efficiency.
Benefits of Thermal Oxidizers
Thermal oxidizers offer several benefits. They are highly effective at reducing emissions of volatile organic compounds (VOCs), which are harmful to human health and the environment. They can also improve energy efficiency by capturing and recycling the heat generated by the combustion process. Another benefit of thermal oxidizers is their ability to operate continuously without requiring frequent maintenance or downtime. This makes them a reliable solution for many manufacturing operations. Additionally, thermal oxidizers can help manufacturers comply with air quality regulations, reducing the risk of fines or legal action. Finally, thermal oxidizers can help companies improve their public image by demonstrating a commitment to environmental responsibility and sustainability. This can be a valuable marketing tool and can help businesses attract environmentally conscious customers.
Applications of Thermal Oxidizers
Thermal oxidizers are used in a variety of industries, including:
Food and Beverage Production
Thermal oxidizers are commonly used in food and beverage production to treat the exhaust gasses generated during the processing and cooking of food products. They are particularly useful in operations that use oil-based cooking processes or that generate large volumes of steam. Thermal oxidizers can help to eliminate odors and VOCs, improving the air quality within the facility and reducing the risk of emissions that could negatively impact the surrounding environment.
Pharmaceutical Manufacturing
Thermal oxidizers are used in the pharmaceutical industry to treat the exhaust gasses generated during the production of drugs and other medical products. They are particularly useful in operations that use solvents or other organic compounds, which can generate harmful emissions. By treating these emissions with a thermal oxidizer, manufacturers can ensure that their operations are compliant with environmental regulations and protect the health of their workers.
Chemical Processing
Thermal oxidizers are widely used in the chemical processing industry to treat the exhaust gasses generated during the manufacturing of chemicals and other products. They are particularly useful in operations that generate high volumes of VOCs or that require high-temperature processing, which can release harmful emissions into the air. Thermal oxidizers can help to eliminate these emissions, improving the air quality within the facility and reducing the risk of negative impacts on the surrounding environment.
Oil and Gas Production
Thermal oxidizers are used in the oil and gas industry to treat the exhaust gasses generated during drilling, refining, and processing operations. They are particularly useful in operations that generate large volumes of VOCs, such as natural gas processing plants or refineries. By treating these emissions with a thermal oxidizer, manufacturers can reduce the risk of harmful emissions that could negatively impact the environment.
Automotive Manufacturing
Thermal oxidizers are used in the automotive industry to treat the exhaust gasses generated during the manufacturing of vehicles. They are particularly useful in operations that involve painting or coating, which can generate harmful emissions. By treating these emissions with a thermal oxidizer, manufacturers can ensure that their operations are compliant with environmental regulations and protect the health of their workers.
Pulp and Paper Production
Thermal oxidizers are used in the pulp and paper industry to treat the exhaust gasses generated during the manufacturing of paper products. They are particularly useful in operations that use solvents or other organic compounds, which can generate harmful emissions. By treating these emissions with a thermal oxidizer, manufacturers can ensure that their operations are compliant with environmental regulations and protect the health of their workers.
Waste Management
Thermal oxidizers are used in the waste management industry to treat the exhaust gasses generated during the processing of waste materials. They are particularly useful in operations that involve incineration or other high-temperature processing, which can generate harmful emissions. By treating these emissions with a thermal oxidizer, waste management companies can reduce the risk of negative impacts on the environment and protect the health of their workers.
Semiconductor Manufacturing
Thermal oxidizers are used in the semiconductor industry to treat the exhaust gasses generated during the production of electronic components. They are particularly useful in operations that use solvents or other organic compounds, which can generate harmful emissions. By treating these emissions with a thermal oxidizer, semiconductor manufacturers can ensure that their operations are compliant with environmental regulations and protect the health of their workers.
Choosing the Right Thermal Oxidizer Manufacturer
To ensure you have the most positive outcome when purchasing a thermal oxidizer from a thermal oxidizer manufacturer, it is important to compare several companies using our directory of thermal oxidizer manufacturers. Each thermal oxidizer manufacturer has a business profile page highlighting their areas of experience and capabilities, along with a contact form to directly communicate with the manufacturer for more information or to request a quote. Review each thermal oxidizer business website using our proprietary website previewer to quickly learn what each company specializes in. Then, use our simple RFQ form to contact multiple thermal oxidizers companies with the same form.




 Air Compressors
Air Compressors  Air Filters
Air Filters Air Pollution Control
Air Pollution Control Blowers
Blowers Dust Collectors
Dust Collectors Industrial Vacuum Cleaning Equipment
Industrial Vacuum Cleaning Equipment Castings & Forgings
Castings & Forgings Bulk Material Handling
Bulk Material Handling Electrical & Electronic Components
Electrical & Electronic Components Flow Instrumentation
Flow Instrumentation Hardware
Hardware Material Handling Equipment
Material Handling Equipment Metal Cutting Services
Metal Cutting Services Metal Forming Services
Metal Forming Services Metal Suppliers
Metal Suppliers Motion Control Products
Motion Control Products Plant & Facility Equipment
Plant & Facility Equipment Plant & Facility Supplies
Plant & Facility Supplies Plastic Molding Processes
Plastic Molding Processes Pumps & Valves
Pumps & Valves Recycling Equipment
Recycling Equipment Rubber Products & Services
Rubber Products & Services